WATER HARDNESS
Hardness is a measure of the amount of certain minerals in water. The amount and proportion of minerals in your brewing water can have a dramatic effect on the flavour of your coffee, as well as affect how likely your espresso machine is to get scaled up, so it’s worth trying to understand a few key concepts.
The term ‘hardness’ originally comes from the effect of minerals in water on soap. The minerals in hard water bind to soap and form an insoluble ‘scum’, making it harder to form a foam, and making the soap less effective in washing. At some point, people noticed that if the water was boiled before use, the water would become less hard, making washing easier. The hardness that could be removed by boiling is referred to as temporary hardness, the hardness that remains, no matter how much you boil the water, is called permanent hardness.
So the definition is easy enough to remember, but to really understand what’s going on, we need to know a little bit about what minerals in water are made of. Each mineral that dissolves in water is made up of ions, which are electrically charged particles. The solid mineral has equal amounts of positive and negative charge mixed together, so the charges cancel each other out. For example, table salt, sodium chloride, is made up of equal parts positively charged sodium (Na+) and negatively charged chloride ions (Cl–). When the mineral dissolves in water, the ions split apart (‘dissociate’) and spread out in the water.
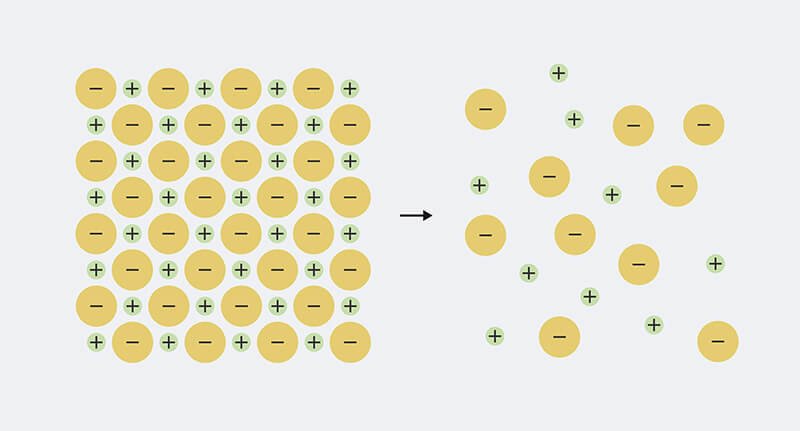
The sodium and chloride in solid table salt are packed tightly together. When it is dissolved in water, it separates into sodium ions (Na+) and chloride ions (Cl–)
Hardness is caused by any mineral where the positively charged ion has more than one charge. The stronger charge in these ions is what causes it to interact with soap — and it is the same charge that means these minerals can help draw flavours out of coffee.
Calcium (Ca2+) and Magnesium (Mg2+) make up nearly all of the hardness in drinking water. Since calcium and magnesium both have two positive charges, they behave in a very similar way, so can be considered interchangeable when we talk about hardness. Some other ions, like iron (Fe3+) or aluminium (Al3+), can technically contribute (WHO 2011), but we normally ignore these, as the amount of them in drinking water should be very low.
So the total hardness, also called ‘general hardness’ or GH, is just a measure of the amount of positively charged calcium and magnesium that is in the water. Because the electrical charge in these ions helps to extract flavour molecules, the GH measurement is an indicator of the extraction power of the water.
Now remember that each positively charged mineral ion has to be paired with negatively charged ions as well. The most important of these in water is bicarbonate (HCO3–). When you boil water with bicarbonate in it, it reacts with any calcium and magnesium in the water to form a solid called limescale (calcium carbonate and magnesium carbonate). So this is what causes temporary hardness — by boiling the water, you are forming limescale, which drops to the bottom. The permanent hardness is whatever hardness is left over — any calcium or magnesium that doesn’t have any bicarbonate to react with.
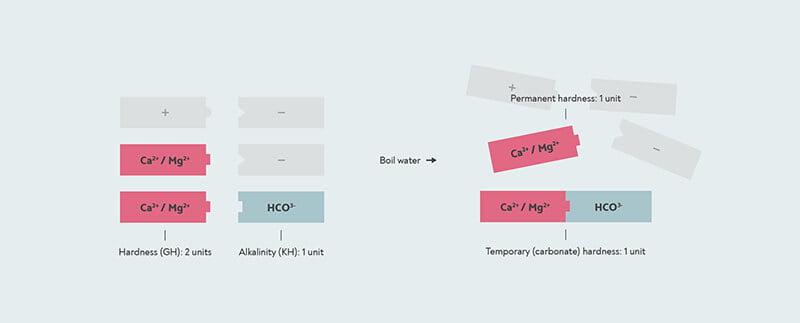
Temporary and permanent hardness. This water has a GH of 2, because there are two calcium/magnesium units (red) in it. It has a temporary hardness of 1, because one carbonate unit binds to one calcium/magnesium to form limescale. The other calcium/magnesium is permanent hardness that doesn’t form limescale and isn’t removed by boiling. The other positive and negative mineral ions in the water are shown in grey.
When we measure the hardness in our water, we usually make two measurements: GH and KH. GH is general hardness, and measures the total amount of calcium and magnesium in the water. KH stands for Karbonathärte, which is the German for Carbonate Hardness. Carbonate hardness is exactly the same as temporary hardness: it means all the hardness that is paired with bicarbonate ions, which can form limescale.
However, where it gets confusing is that a typical KH test doesn’t actually measure carbonate hardness. A KH drop test just measures the amount of bicarbonates in the water, also called the alkalinity. This measure is also important because the alkalinity buffers out acidity in the coffee, and makes it taste less sour. Too much alkalinity will make the coffee taste flat.
In our example so far, the alkalinity is the exact same as the temporary hardness, because there is enough calcium and magnesium to react with all of the bicarbonate and form limescale. This is the case in most natural drinking water — the alkalinity and the carbonate hardness or temporary hardness are the exact same, so most of the time the KH test does also tell you the carbonate hardness.
However, in some cases, the amount of bicarbonate can be more than the amount of calcium or magnesium. This can happen when the water has been softened, or when salt water has got into the drinking water. In this case, there might not be enough calcium and magnesium to react with all of the bicarbonate. With this kind of water, if you boil it, all the hardness is removed, so the temporary hardness is the same as the total hardness, and there is no permanent hardness at all.
In this case, the KH test will give you a higher result than your GH. When the result of a KH test is higher than the GH, then the carbonate hardness and the general hardness are actually the same. The number the KH test gives you is then actually the alkalinity, not the carbonate hardness.
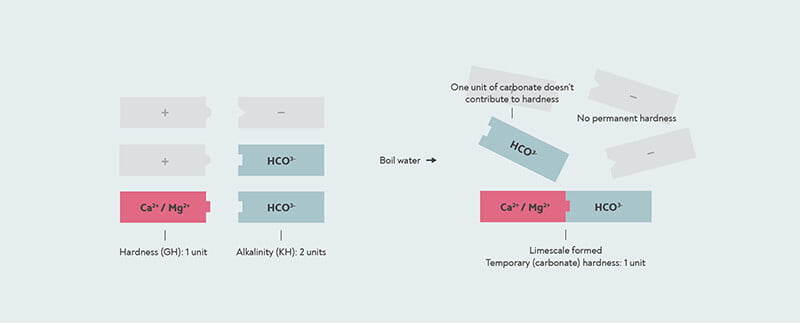
When the alkalinity is higher than the general hardness, then all the hardness is temporary or carbonate hardness. In this case, the remaining alkalinity does not contribute to hardness. The KH drop kit measures an alkalinity of 2, but the carbonate hardness is actually only 1, because there is only 1 unit of hardness for it to form limescale with.
This case is rare enough in tap water, though, that most of us can ignore it when we’re testing our water. In most drinking water, KH = alkalinity = temporary hardness. Permanent hardness is just whatever hardness is left over, so: Permanent Hardness = GH − KH.
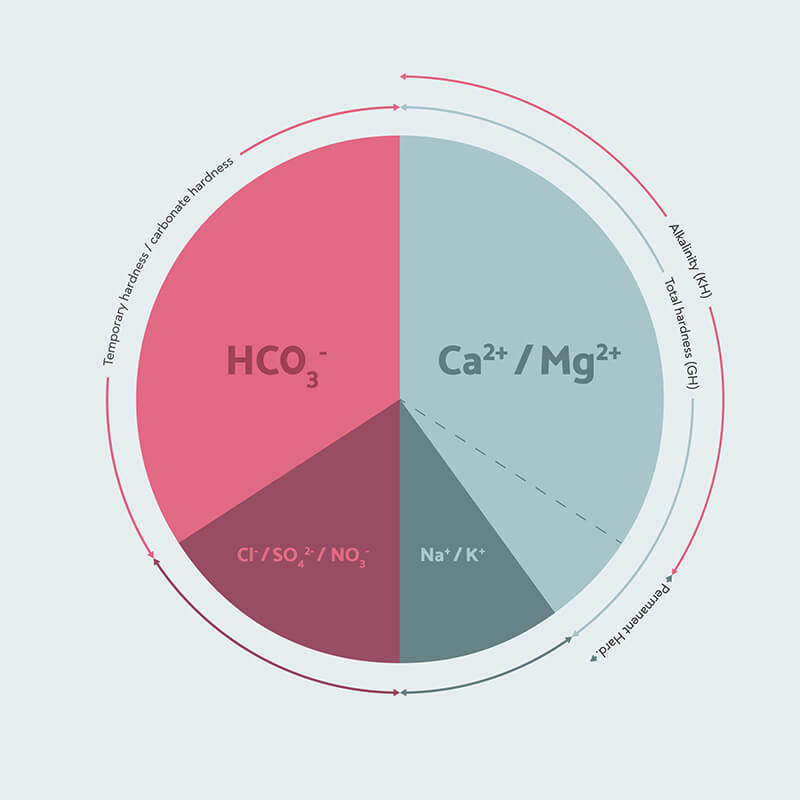
In summary, these are the three measures that really matter in coffee brewing: the total hardness or general hardness (GH), the temporary hardness, or carbonate hardness, and the alkalinity. The total hardness helps the water to extract flavour from the coffee. The temporary hardness is what determines how much of that hardness can form limescale. Finally, the alkalinity determines how much the water will remove acidity from the coffee.
If the KH is below GH, then the KH test gives you the figure for both the temporary hardness and the alkalinity, because KH determines how much of the GH can form limescale. If the KH is above GH though, then the KH just measures the alkalinity, and the temporary hardness is just the same as the total hardness, because the ‘extra’ KH can’t form limescale by itself.
Source: Barista Hustle
Link: https://www.baristahustle.com/blog/water-hardness/















